Designation process of MDR/IVDR Notified Bodies - update · MDlaw – Information platform on European medical device regulations
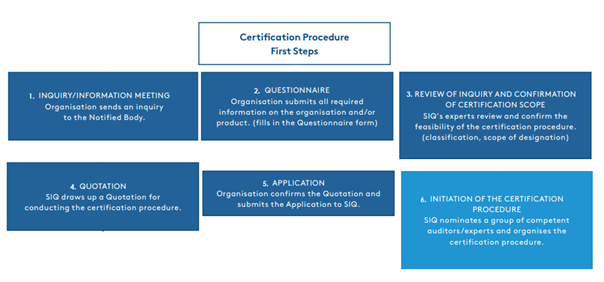
Procedure for Certification of Medical Devices in Accordance with the Regulation (EU) 2017/745 on Medical Devices (MDR) - SIQ
![Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses](https://learngxp.com/wp-content/uploads/2021/04/ELM-320-01-Requirements-Relating-to-Notified-Bodies-for-EU-MDR.png)
Requirements Relating to Notified Bodies for EU MDR [Video] - LearnGxP: Accredited Online Life Science Training Courses

EU Commission Release Information on the Applications for Designation as a Notified Body | Advena.mt