Scale Up and Post Approval Changes | SUPAC | Regulatory Affairs | DRA | Pharmaceutics | Pharma Wins - YouTube

SCALE UP AND POSTAPPROVAL CHANGES (SUPAC) GUIDANCE FOR INDUSTRY: A REGULATORY NOTE | Semantic Scholar

Scale Up and Postapproval Changes (Supac) Guidance For Industry: A Regulatory Note | PDF | Pharmacokinetics | Medicine

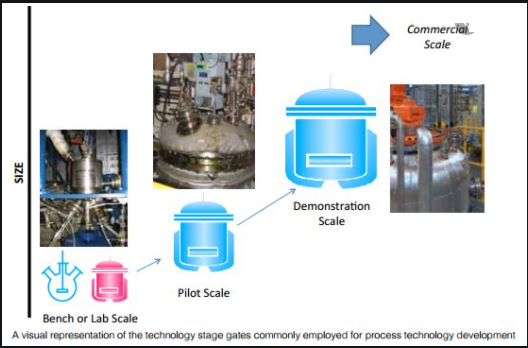
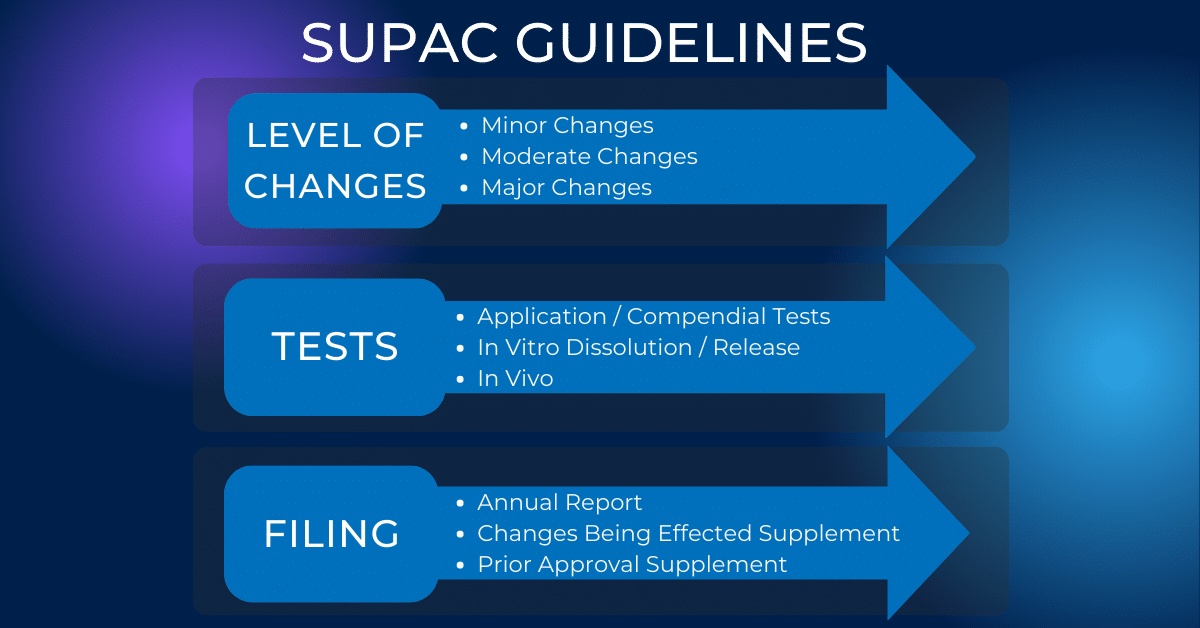
Best Practices for the Development, Scale-up, and Post-approval Change Control of IR and MR Dosage Forms in the Current Quality-by-Design Paradigm | AAPS PharmSciTech

Learn how SUPAC guidance documents can accelerate your drug program. Read more here: https://ow.ly/3nH250PWxe4 | Certara posted on the topic | LinkedIn